How can something as simple as a grain of salt clear entire roads and make it possible to get around safely in the winter?
In essence, salt lowers the freezing point of water. It prevents the formation of the bond between pavement and snow or ice so that roads can be cleared more quickly and easily, making them safer for driving.
How It Works
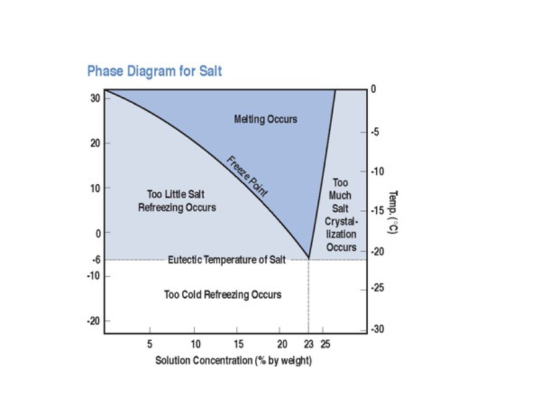
First, salt is spread on the snow or ice-covered road. As the salt particles come into contact with the snow or ice, melting begins and water is produced. This water containing dissolved salt is called “brine.” Brine freezes at lower temperatures than regular water, so it remains a liquid at below-freezing temperatures.
The brine works its way further into the snow and ice and eventually down to the road surface. From here, brine can spread out under the ice, breaking the bond between the road surface and the ice. The remaining snow and ice floats on top of the liquid brine, allowing traffic to easily break it down into slush. Finally, snow plows can move the slush to the side of the road and clear the way for safer, more drivable roadways.
You can actually speed up this process by pre-wetting salt with salt brine, which can accelerate its melting action.
